How to Implement ST108: Checklist for Selecting an ST108 Compliant Water Processing System
Choosing the right water treatment system is critical for hospitals, surgery centers, and sterile processing departments working to meet ANSI/AAMI ST108 standards. The wrong system or one that isn’t carefully monitored can lead to compliance failures, costly downtime, and risk to patient safety. Before selecting or upgrading your water system, your facility should evaluate the following:
Know Your Municipal Water Supply:
- Yearly chemical fluctuations and seasonal variations
- Microbial levels throughout the year
- Organic and inorganic contaminant levels
- Details of the municipal water treatment process and prospective monitoring methods
- Temperature ranges across all seasons
Confirm Facility Readiness:
- Piping selected to align with disinfectant type used by the municipality
- Water quality requirements verified for each washer-disinfector, sterilizer, and reprocessor
- Water quality requirements confirmed for all medical devices being processed
- Ability to monitor water quality at each stage of processing
ST108 Pretreatment Equipment Monitoring
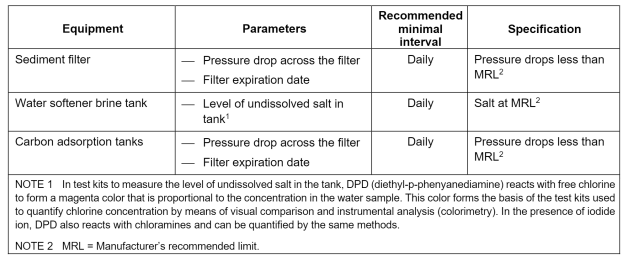
Plan for Ongoing Compliance:
- Routine audits conducted with current water quality data and reporting
- A designated individual responsible for interpreting audit data and overseeing water quality
- System evaluated for future expansion and capacity needs
- A written contingency plan in place including criteria for system shutdown and safe restart procedures
Implementing ST108 requires a proactive approach to water quality management—from initial testing and treatment to ongoing monitoring and maintenance. These steps help facilities meet the standard’s requirements and protect both patients and equipment.
Partnering for Long-Term ST108 Compliance
Meeting AAMI ST108 requirements isn’t a one-time implementation project; it’s an ongoing commitment to water quality, patient safety, and instrument protection. Even after a compliant system is installed, facilities must maintain strict monitoring, documentation, and preventive service to stay inspection-ready.
At Western Reserve Pure Water, we provide hospitals, surgical centers, and sterile processing departments in Greater Cleveland and Northeast Ohio with:
- On-site water testing and validation to confirm conductivity, microbial, and endotoxin levels meet ST108 standards
- Preventive maintenance programs that reduce downtime and extend equipment life
- Compliance support and reporting tools to simplify audits and survey readiness
- Scalable solutions designed to grow with your facility’s needs

Filtration Systems Designed for ST108 Compliance
Western Reserve Pure Water’s filtration systems are built to help healthcare facilities meet the demanding water quality standards of ST108. Designed for reliability and performance, these systems make sure your water is safe, clean, and ready for everything from medical device reprocessing to everyday use.

End-To-End Membrane Cleaning – Available Nationwide!
Save your time, money and effort by having Western Reserve Pure Water clean your existing membrane. We offer short turnaround time and loaners if needed. Shipping is an easy process, and we offer service and maintenance plans. We have customers anywhere across the U.S.
Implementing ST108 FAQs
Maintaining RO and DI membranes is critical to meeting ST108 bacterial and endotoxin limits. While many service providers offer membrane cleaning, look for companies that provide validated cleaning protocols, monitoring of bacterial counts before and after service, and certification of compliance with ST108 standards. At Western Reserve Pure Water, our service programs ensure membranes are properly maintained to consistently meet ST108 water quality requirements.
Switching to a central DI or RO/DI water system can deliver significant ROI. Central systems reduce reliance on expensive bottled water, minimize handling risks, and ensure consistent ST108-critical water quality throughout the sterile processing department. Over time, facilities often see cost savings, reduced labor, fewer compliance issues, and longer instrument life.
Rapid endotoxin testing is essential to verify water quality in sterile processing. Western Reserve Pure Water partners with local labs and provides on-site testing options for healthcare facilities in Greater Cleveland and Northeast Ohio, helping teams quickly confirm compliance with ST108 endotoxin limits and address any deviations.
AAMI periodically revises ST108 to reflect new research and technology. To prepare, facilities should maintain detailed system documentation, perform regular audits, and ensure flexibility in RO/DI design so membranes, resin, and monitoring equipment can be upgraded as standards evolve. Staying proactive reduces disruption and keeps the facility compliant with any updated ST108 requirements.
Integration includes assessing current water quality, retrofitting or installing RO/DI systems, establishing monitoring protocols, training staff, and creating a maintenance and validation schedule. Following ST108 ensures consistent critical water quality for final rinses and steam generation.
Validation involves documenting system performance under normal operating conditions, including microbial counts, endotoxin levels, conductivity, and resistivity. Western Reserve provides guidance and validation support to ensure your facility meets ST108 standards.
Need Help Navigating ST108?
Don’t leave compliance to chance. Contact Western Reserve Pure Water to assess your current systems and get expert recommendations.

