Protecting Medical Instruments and Patients with ST108 Water Standards
Beyond meeting purity specifications, water quality directly impacts the life of surgical instruments and the safety of every procedure. Even trace amounts of minerals, chlorine, or bacteria can cause corrosion, spotting, or biofilm buildup, leading to premature instrument replacement and costly downtime in sterile processing departments.
Under AAMI ST108, regular monitoring of conductivity, bacterial levels, and endotoxins ensures water is performing as required—not just during system startup, but throughout daily medical use.
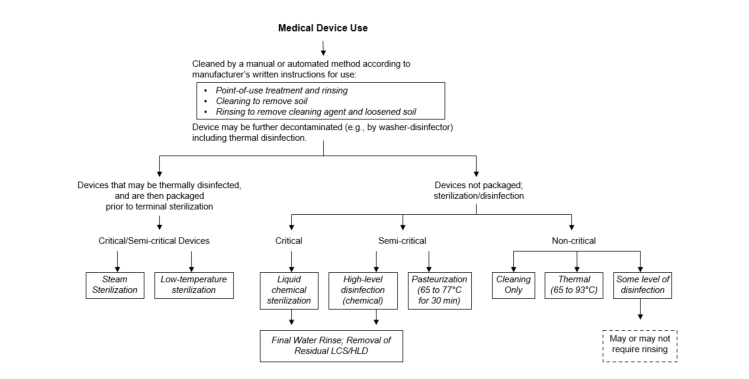
Consistent testing and documentation also provide a compliance trail that surveyors and accrediting bodies look for during hospital inspections.
At Western Reserve Pure Water, we design ST108-compliant systems that not only deliver the required water quality but also include monitoring solutions, validation support, and service programs. This approach keeps sterile processing teams in Greater Cleveland and Northeast Ohio confident that every cycle is backed by safe, reliable water.
Extending Equipment Life and Reducing Costs
The role of water in sterile processing doesn’t just protect patients—it protects your investment. Washers, sterilizers, and distribution piping are highly sensitive to minerals, hardness, and chlorine present in untreated water. Over time, these impurities cause scale buildup, rust, and premature equipment failure, driving up repair costs and downtime.
By following AAMI ST108 water quality standards, healthcare facilities reduce wear and tear on both instruments and processing equipment. High-purity water prevents residue accumulation, keeps heating elements and spray arms functioning properly, and reduces the frequency of costly service calls.
For hospitals and surgical centers in Greater Cleveland and Northeast Ohio, Western Reserve Pure Water provides end-to-end water treatment solutions that not only meet ST108 standards but also deliver measurable ROI by extending the life of critical sterile processing equipment.

Filtration Systems Designed for ST108 Compliance
Western Reserve Pure Water’s filtration systems are built to help healthcare facilities meet the demanding water quality standards of ST108. Designed for reliability and performance, these systems make sure your water is safe, clean, and ready for everything from medical device reprocessing to everyday use.

End-To-End Membrane Cleaning – Available Nationwide!
Save your time, money and effort by having Western Reserve Pure Water clean your existing membrane. We offer short turnaround time and loaners if needed. Shipping is an easy process, and we offer service and maintenance plans. We have customers anywhere across the U.S.
ST108 Water in Sterile Processing FAQs
Deionization (DI) resin plays a key role in producing critical water for sterile processing. As resin becomes exhausted, it loses its ability to remove charged ions, which lowers the water’s resistivity and raises conductivity. Under AAMI ST108, falling below the resistivity threshold means the water no longer meets the required purity for final rinsing or steam generation. Regular monitoring and timely resin replacement are essential to stay compliant.
ST108 specifies strict conductivity limits for critical water, generally measured in microsiemens per centimeter (µS/cm), to ensure minimal dissolved solids are present. High-quality, well-maintained DI resin ensures conductivity stays within these limits, while poor-grade or exhausted resin can cause spikes that risk noncompliance. Western Reserve Pure Water provides DI systems designed to maintain stable conductivity levels aligned with ST108 requirements.
Reverse osmosis (RO) systems are highly effective at reducing endotoxins, but performance can drift over time due to membrane fouling, scaling, or biofilm growth. ST108 recommends routine monitoring of bacterial and endotoxin levels, typically through scheduled lab testing and in-house checks. Most healthcare facilities test RO water monthly at minimum, with additional testing during system validation, after maintenance, or if a compliance issue is suspected. Consistent testing ensures early detection of problems and protects patients from infection risk.
ST108 requires that final rinse water meet critical water standards, meaning it must be extensively treated to remove minerals, bacteria, and endotoxins. This ensures that no harmful contaminants are left behind on instruments that will contact sterile body sites. Compliance with this standard is essential for patient safety and survey readiness.
Even trace impurities in water, such as chlorine, hardness, or microbial contaminants can cause pitting, spotting, and corrosion on surgical instruments. By following ST108 guidelines for water quality, healthcare facilities can significantly extend instrument life, reduce repair costs, and maintain compliance during inspections.
Need Help Navigating ST108?
Don’t leave compliance to chance. Contact Western Reserve Pure Water to assess your current systems and get expert recommendations.

